Time, pH, and size dependency of silver nanoparticle dissolution: the road to equilibrium†
Abstract
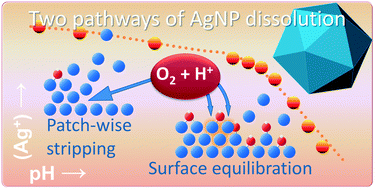
Oxidative dissolution has large implications for the environmental fate and toxicity of silver nanoparticles (AgNPs). In this study, we quantify the kinetics, pH, and size dependency of silver ion (Ag+) release from AgNPs and explain our results in a consistent manner with a mechanistic view. Pristine AgNPs are covered by partially oxidized silver present in a single layer of subvalent ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ag3OH groups that will be released by oxidative dissolution via two different pathways. Undersaturation of a solution, created by acidification, will initiate a fast oxidative dissolution process in which a pristine surface can be opened at particular points that grow laterally until a full layer of Ag is stripped off. At the newly exposed surface,
Ag3OH groups that will be released by oxidative dissolution via two different pathways. Undersaturation of a solution, created by acidification, will initiate a fast oxidative dissolution process in which a pristine surface can be opened at particular points that grow laterally until a full layer of Ag is stripped off. At the newly exposed surface, ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ag3OH is reformed. The opening of new spots stops due to increasing Ag+ concentrations. Via another pathway, the initial
Ag3OH is reformed. The opening of new spots stops due to increasing Ag+ concentrations. Via another pathway, the initial ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ag3OH can be released by oxidative dissolution while simultaneously a new stable surface state is built with subvalent silver in two layers. This process is initiated by dilution and is visible around neutral pH values and may release a maximum of 30 ± 1 μmol Ag+ m−2. Its equilibration can be well described with a formulated thermodynamic model. The equilibrium constant (log K) is linearly related to the specific surface area of the AgNPs used, but can be shifted by the type of capping agent. The particle size dependency of the log K can be attributed to a surface Gibbs free energy contribution of 0.7 ± 0.1 J m−2. Ag+ release by stripping is relatively fast (∼1 day) in contrast to the process that leads to equilibration of two types of surface species that differ in the amount of subvalent silver. For this process, a kinetic Langmuir model has been developed in which the rate of Ag+ release is governed by adsorbed molecular oxygen that can be activated via a proton, while adsorption of molecular oxygen by itself can become rate limiting in the initial stage of dissolution with high rates of release. In our study with data of different kinds, the overall release and equilibration by AgNPs has been interpreted successfully with a coherent, overarching mechanistic view.
Ag3OH can be released by oxidative dissolution while simultaneously a new stable surface state is built with subvalent silver in two layers. This process is initiated by dilution and is visible around neutral pH values and may release a maximum of 30 ± 1 μmol Ag+ m−2. Its equilibration can be well described with a formulated thermodynamic model. The equilibrium constant (log K) is linearly related to the specific surface area of the AgNPs used, but can be shifted by the type of capping agent. The particle size dependency of the log K can be attributed to a surface Gibbs free energy contribution of 0.7 ± 0.1 J m−2. Ag+ release by stripping is relatively fast (∼1 day) in contrast to the process that leads to equilibration of two types of surface species that differ in the amount of subvalent silver. For this process, a kinetic Langmuir model has been developed in which the rate of Ag+ release is governed by adsorbed molecular oxygen that can be activated via a proton, while adsorption of molecular oxygen by itself can become rate limiting in the initial stage of dissolution with high rates of release. In our study with data of different kinds, the overall release and equilibration by AgNPs has been interpreted successfully with a coherent, overarching mechanistic view.


 Please wait while we load your content...
Please wait while we load your content...