Photoenhanced transformation of hydroxylated fullerene (fullerol) by free chlorine in water†
Abstract
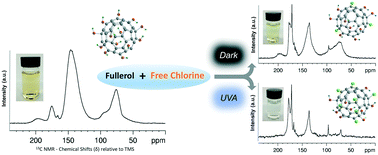
Water-soluble, oxidized fullerenes, termed as fullerols or fullerenols, have gained increasing attention as they have been identified as primary daughter product(s) when C60 is exposed to ubiquitous, reactive (oxidative) environmental scenarios including UV light (including sunlight UVA), radical oxygen species (ROS), and ozone. However, subsequent and resulting environmental reactivity of such fullerol (daughter) products remains poorly defined. Here, we examine the chemical and physical transformation of a model fullerol in the presence of free chlorine, a globally applied oxidant and disinfectant. Under both photoexcited and ground state (dark) conditions with free chlorine, fullerol reaction kinetics are described for batch systems over a range of environmentally relevant conditions. Resulting fullerene-based (here as a 60 carbon cage) oxy-chlorinated products were characterized and described with a suite of analytical techniques, including 13C-NMR, FTIR, XPS, and UV-vis spectroscopy. Product physical (behavior) properties are described through quartz crystal microbalance (QCM) and classic water–octanol partition experiments. For all conditions evaluated in the presence of free chlorine, fullerol readily reacts, resulting in significant surface (functional) alteration, leading to significantly different physical behaviors when compared to parent materials. Further, the presence of light (UVA, 351 nm) was observed to significantly enhance reaction rates and product oxidation extent for all scenarios.

 Please wait while we load your content...
Please wait while we load your content...