Photoenhanced oxidation of C60 aggregates (nC60) by free chlorine in water†
Abstract
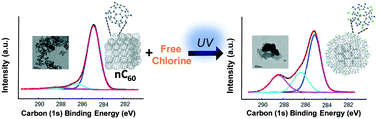
While there have been a number of fundamental studies focused on the physical and biological behaviors of C60 aggregates (nC60) in model environmental and engineered systems, the aqueous reactivity of C60 (as nC60) is much less understood and remains a critical gap in accurate life cycle modeling. In this study, facile, photo-enhanced physicochemical transformations of nC60 in the presence of free chlorine, a globally applied disinfectant, are quantitatively described. Batch reaction kinetics between nC60 and free chlorine are explored and modeled for both the ground state (dark conditions) and the photo-excited state (light irradiation conditions, UVA) over a matrix of environmentally relevant conditions (e.g., redox potentials, dissolved oxygen, light irradiation). The resulting products are described using a suite of spectroscopy techniques, including FTIR, XPS, and UV-vis, demonstrating oxidized fullerene products with varied covalent addition(s) of oxygen and chlorine atoms depending on the reaction conditions. For all conditions, UVA light irradiation (351 nm, 2 mW cm−2) significantly enhances the oxidation of nC60 by free chlorine, as indicated by both higher reaction rates and product(s) oxidation extent. Aggregation kinetic studies indicate that the resulting oxidized products, as soft clusters, are relatively more stable, compared to the parent nC60, upon photo-oxidation in the presence of monovalent (NaCl) and divalent (MgCl2) ions (via critical coagulation concentration evaluations). Further, increased product surface hydrophilicity is also demonstrated through classic water–octanol partition (Kow) experiments.
- This article is part of the themed collection: Sustainable Nanotechnology Organization 2015

 Please wait while we load your content...
Please wait while we load your content...