A low-noble-metal W1−xIrxO3−δ water oxidation electrocatalyst for acidic media via rapid plasma synthesis†
Abstract
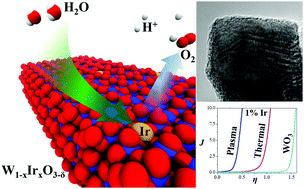
Acid-based electrolysis has many advantages, but to achieve simultaneous activity and stability, commercial water oxidation catalysts rely on noble metal oxides that are expensive and too rare for the global scale. Here, earth-abundant tungsten was used as a structural metal to dilute the noble metal iridium content while maintaining high activity and stability in acid. Mixed-metal oxide catalysts were synthesized using rapid plasma oxidation in which the non-equilibrium reaction environment permitted better formation of a homogenous W1−xIrxO3−δ phase. With an Ir metal content as low as 1%, a competitive and durable overpotential for oxygen evolution was achieved. Relative to high Ir content, low Ir compositions consisted of a more highly crystalline, phase-pure iridium polytungstate which was more catalytically active per Ir content. Moreover, the plasma-synthesized material had a sharp electrocatalytic improvement over an equivalent composition synthesized via standard thermal oxidation, demonstrating the value of non-equilibrium synthesis to find new catalysts.


 Please wait while we load your content...
Please wait while we load your content...