Development of novel inorganic electrolytes for room temperature rechargeable sodium metal batteries†
Abstract
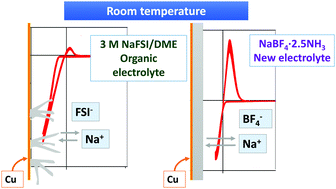
Rechargeable sodium metal batteries have attracted attention as promising power sources over the last several years, because they are a lower cost option for large scale energy storage from the electric grid. However, among the main problems of Na-metal batteries, the formation of nonuniform solid electrolyte interfaces and the dendritic growth of sodium metal are particularly challenging. Here, we report on highly-concentrated sodium electrolytes based on liquid ammonia in which a large amount of sodium salt has been dissolved. These electrolytes can be generally described as NaY·xNH3, where Y is the anion (I−, BF4− or BH4−) and x indicates the molar ratio of ammonia to sodium salt. These electrolytes are characterized by their excellent properties such as low flammability and high specific conductivity. Most importantly, they support nondendritic and highly reversible plating–stripping of sodium, paving the way for the development of sodium metal anodes at room temperature. In addition, sodium deposition can be performed on copper as a substrate with a high coulombic efficiency and deposit stability.


 Please wait while we load your content...
Please wait while we load your content...