Partial indium solubility induces chemical stability and colossal thermoelectric figure of merit in Cu2Se†
Abstract
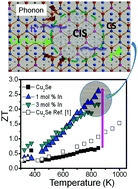
High thermoelectric figure of merit, ZT ∼ 2.1 at 1000 K, have been reported in Cu2−xSe-based materials. However, their deployments in operational devices have been hampered by chemical instability and low average ZT (ZTave) values. Here, we demonstrate improved chemical stability and a record high ZTave ∼ 1.5 over a broad temperature range (T ≤ 850 K) in Cu2Se/CuInSe2 nanocomposites, with ZT values ranging from 0.6 at 450 K to an unprecedentedly large value of 2.6 at 850 K for the sample with 1 mol% In. This remarkable performance is attributed to the localization of Cu+ ions induced by the incorporation of In into the Cu2Se lattice, which simultaneously boost the electrical conductivity and reduce the thermal conductivity of the nanocomposites. These findings pave the way for large-scale utilization of Cu2Se-based materials in thermoelectric generators.


 Please wait while we load your content...
Please wait while we load your content...