Compatibility issues between electrodes and electrolytes in solid-state batteries†
Abstract
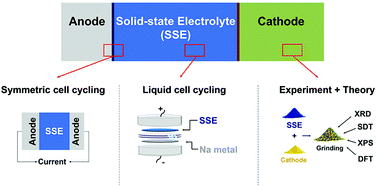
Remarkable success has been achieved in the discovery of ceramic alkali superionic conductors as electrolytes in solid-state batteries; however, obtaining a stable interface between these electrolytes and electrodes is difficult. Only limited studies on the compatibility between electrodes and solid electrolytes have been reported, partially because of the need for expensive instrumentation and special cell designs. Without simple yet powerful tools, these compatibility issues cannot be systematically investigated, thus hindering the generalization of design rules for the integration of solid-state battery components. Herein, we present a methodology that combines density functional theory calculations and simple experimental techniques such as X-ray diffraction, simultaneous differential scanning calorimetry and thermal gravimetric analysis, and electrochemistry to efficiently screen the compatibility of numerous electrode/electrolyte pairs. We systemically distinguish between the electrochemical stability of the solid-state conductor, which is relevant wherever the electrolyte contacts an electron pathway, and the electrochemical stability of the electrode/electrolyte interfaces. For the solid electrolyte, we are able to computationally derive an absolute thermodynamic stability voltage window, which is small for Na3PS4 and Na3PSe4, and a larger voltage window which can be kinetically stabilized. The experimental stability, when measured with reliable techniques, falls between these thermodynamic and kinetic limits. Employing a Na solid-state system as an example, we demonstrate the efficiency of our method by finding the most stable system (NaCrO2|Na3PS4|Na–Sn) within a selected chemical space (more than 20 different combinations of electrodes and electrolytes). Important selection criteria for the cathode, electrolyte, and anode in solid-state batteries are also derived from this study. The current method not only provides an essential guide for integrating all-solid-state battery components but can also significantly accelerate the expansion of the electrolyte/electrode compatibility data.
- This article is part of the themed collection: 2017 Energy and Environmental Science HOT articles


 Please wait while we load your content...
Please wait while we load your content...