Green synthesis of a new layered aluminium citraconate: crystal structures, intercalation behaviour towards H2O and in situ PXRD studies of its crystallisation†
Abstract
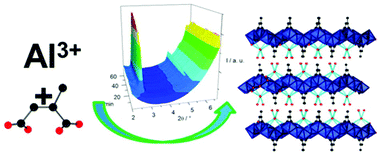
A new Al-based layered MOF [Al2(OH)4(O2C-C3H4-CO2)]·nH2O denoted as CAU-15-Cit was synthesised under mild aqueous conditions. It exhibits a layered structure incorporating infinite chains of edge-sharing AlO6 polyhedra being interconnected by citraconate anions to arrange into layers, which are stacked in an AAA fashion (citraconic acid = methylmaleic acid = H2Cit, HO2C-C3H4-CO2H). The crystal structures of the hydrated and dehydrated MOF were determined ab initio from powder X-ray diffraction (PXRD) data. The hydrated form of this compound (n ≈ 3) crystallises in the space group C2/c (a = 7.4074(8), b = 23.006(3), c = 7.0890(4) Å, β = 85.024(7)°) and is converted to a triclinic anhydrous form (n = 0) upon dehydration (a = 7.0010(3), b = 7.5062(8), c = 9.2212(8) Å, α = 72.143(7), β = 88.617(9), γ = 85.242(8)°, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) in which the layers are interdigitated with a decreased interlayer distance. Physisorption measurements of the anhydrous form indicated no porosity towards nitrogen but an uptake of water vapour was measured showing a sigmoidal adsorption curve and a capacity of ≈24 wt%. This is in good agreement with the theoretical capacity for complete intercalation (≈25 wt%). Based on the temperature dependent PXRD data of the hydrated form, the intercalated water is removed at around 100 °C and the framework decomposes above 350 °C. The dehydration process was further compared to the behaviour of the structurally related aromatic variant of the MOF based on phthalic acid, denoted as CAU-15 [Al2(OH)4(O2C-C6H4-CO2)]·nH2O. The crystallisation of CAU-15-Cit was investigated by means of in situ PXRD measurements during the synthesis using synchrotron radiation at temperatures between 90 and 130 °C. Evaluation of the kinetics using the Sharp–Hancock method clearly indicated different kinetic regimes for the reactions, regardless of the synthesis temperature. At lower temperatures the rate limiting step during the initial period is nucleation, while this initial reaction stage is kinetically limited by diffusion at higher temperatures. The second reaction stage at all temperatures is approximately of the first order.
) in which the layers are interdigitated with a decreased interlayer distance. Physisorption measurements of the anhydrous form indicated no porosity towards nitrogen but an uptake of water vapour was measured showing a sigmoidal adsorption curve and a capacity of ≈24 wt%. This is in good agreement with the theoretical capacity for complete intercalation (≈25 wt%). Based on the temperature dependent PXRD data of the hydrated form, the intercalated water is removed at around 100 °C and the framework decomposes above 350 °C. The dehydration process was further compared to the behaviour of the structurally related aromatic variant of the MOF based on phthalic acid, denoted as CAU-15 [Al2(OH)4(O2C-C6H4-CO2)]·nH2O. The crystallisation of CAU-15-Cit was investigated by means of in situ PXRD measurements during the synthesis using synchrotron radiation at temperatures between 90 and 130 °C. Evaluation of the kinetics using the Sharp–Hancock method clearly indicated different kinetic regimes for the reactions, regardless of the synthesis temperature. At lower temperatures the rate limiting step during the initial period is nucleation, while this initial reaction stage is kinetically limited by diffusion at higher temperatures. The second reaction stage at all temperatures is approximately of the first order.


 Please wait while we load your content...
Please wait while we load your content...