Isostructural lanthanide-based metal–organic frameworks: structure, photoluminescence and magnetic properties†
Abstract
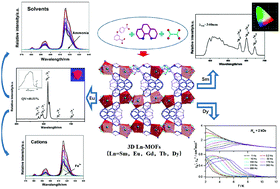
A series of the anhydrous lanthanide-based metal–organic frameworks (Ln-MOFs) were successfully synthesized under hydrothermal conditions of Ln(III) ions with 3,4′-oxybis(benzoate) (3,4′-oba), oxalate (ox) and 1,10-phenanthroline (phen). Structural analyses revealed that they are isostructural 3D open-frameworks with the formula of [Ln(3,4′-oba) (phen)(ox)0.5]n (Ln = Sm for 1, Eu for 2, Gd for 3, Tb for 4, and Dy for 5) and the topology of {33·43·58·6}. Within the 3D structure, the [LnO6N2] units are bridged by ox to form [Ln2ox] dimers and then are further connected by 3,4′-oba ligands. These complexes feature high chemical stabilities in common solvents, boiling water, and acidic (pH = 3) and alkaline (pH = 13) solutions and high thermal stability even up to 400 °C. The photoluminescence and magnetic properties were studied. Especially, 2 shows bright red emission with a high luminescence efficiency of 40.51%. We mainly discuss the luminescence properties of 2 in common solvents and inorganic ions. It shows luminescence stability in common solvents and the ability to detect ammonia and Fe3+ ions under a long excitation wavelength (350 nm). Significantly, magnetic studies reveal that the Dy-MOF, 5, shows a typical single-molecule magnetic behavior. Under zero dc field, compound 5 shows temperature and frequency dependent out-of-phase (χ′′M) signals, indicating the existence of slow magnetic relaxation.


 Please wait while we load your content...
Please wait while we load your content...