Characterization of ammonia binding to the second coordination shell of the oxygen-evolving complex of photosystem II†
Abstract
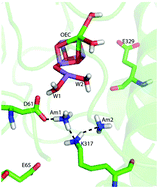
The second-shell ammonia binding sites near the OEC (oxygen-evolving complex) of PSII are characterized by combined Continuum Electrostatic/Monte Carlo (MCCE), QM/MM and DFT calculations and compared with new and earlier experimental measurements. MCCE shows ammonia has significant affinity at 6 positions but only two significantly influence the OEC. Although the pKa of ammonium ion is 9.25, it is calculated to only bind as NH3, in agreement with its low affinity at low pH. The calculations also help explain the experimentally observed competitive binding of ammonia with chloride. Ammonia and Cl− compete for one site. Electrostatic interactions cause Cl− to effect ammonia at two other sites. Cl− stabilizes the multiline g = 2.0 form of the S2 state (OEC Mn oxidation state 3444) while ammonia only binds in the g = 4.1 form of the S2 state (oxidation state 4443) due to the movement of the positive charge between Mn1 and Mn4. One ammonia binds near Mn4 and shares a proton with D2-K317, making the ion pair NH4+K3170D61−, making ammonia binding sensitive to the K317A mutation. The affinity of ammonia is also dependent on the protonation state of water 2, a primary ligand to Mn4.


 Please wait while we load your content...
Please wait while we load your content...