Regioisomerism in coordination chemistry: oxidative addition of a bifunctional ligand to palladium, stabilized with 1,2-bis(diphenylphosphino)ethane†
Abstract
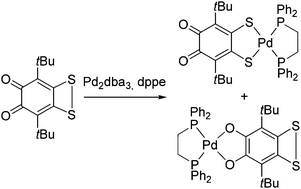
A unique phenomenon of regioisomerism in coordination chemistry was discovered: the reaction of a sterically hindered o-quinone annelated with a dithiete ring with Pd2dba3 in the presence of 1,2-bis(diphenylphosphino)ethane (dppe) gave a mixture of two regioisomers: catecholate 3Cat and dithiolate 3Dit. Both isomers were isolated in crystalline form and characterized by NMR, IR and X-ray diffractometry studies. DFT calculations reveal that the 3Dit species is more thermodynamically stable than the isomer 3Cat. Isomerization of 3Cat to 3Dit in solution was observed.


 Please wait while we load your content...
Please wait while we load your content...