Unusual extraction of trivalent f-cations using diglycolamide dendrimers in a room temperature ionic liquid: extraction, spectroscopic and DFT studies†
Abstract
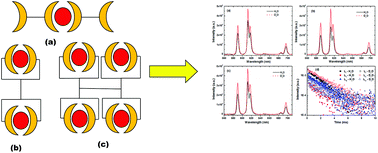
The complexation of Am3+ and Eu3+ was studied with three generations of diglycolamide (DGA)-functionalized poly(propylene imine) diaminobutane dendrimers (DGA-Den) with two, four and eight DGA moieties by solvent extraction and luminescence spectroscopy in a room temperature ionic liquid, viz. 3-butyl-1-methyl imidazolium bis(trifluoromethanesulfonyl)imide ([C4mim][Tf2N]). The extraction of trivalent f-cations was found to increase with decreasing HNO3 concentrations conforming to a cation exchange mechanism with the extraction of Eu3+ being higher than that of Am3+ in the acid concentration range of 0.01–6.0 M HNO3. The nature of the extracted species showed unusual trends compared to those reported previously in molecular diluents. Fluorescence lifetime data suggested the absence of H2O in the extracted complexes meaning strong inner-sphere complexes. The nature of the extracted complexes predicted by solvent extraction was supported by DFT computations.


 Please wait while we load your content...
Please wait while we load your content...