BODIPY dyes with thienyl- and dithienylthio-substituents – synthesis, redox and fluorescent properties†
Abstract
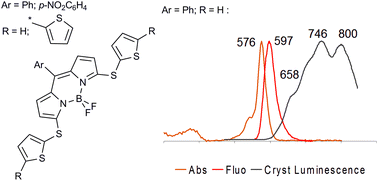
8-Phenyl- and 8-(4-nitrophenyl)-BODIPYs with thien-2-ylthio- and (2,2′-dithien-5-yl)-thio-substitution at the 3,5-positions were synthesized. 2-Thienylthio derivatives were obtained using two different sequences, i.e., via nucleophilic substitution in the corresponding 1,9-dichlorodipyrromethenes, followed by BODIPY formation and via the same reaction using 3,5-dichloro-BODIPY dyes. The “dipyrromethene route” was observed to result in better overall yields. All the dyes were characterized by UV-Vis and fluorescence spectroscopy as well as cyclic voltammetry (CVA) studies. The UV-Vis spectra exhibited slight dependence on the thiophene chain length. The thienylthio derivatives fluoresce with modest quantum yields; conversely, no fluorescence has been detected for their dithienylthio counterparts. 8-Phenyl-3,5-di(thien-2-ylthio)-BODIPY was characterized by X-ray crystallography, which showed the layered arrangement of the molecules. The thienyl fragments of different molecules in the same layer form pairs alike H-aggregates, whereas the BODIPYs moieties in the different layers are arranged in a J-aggregate fashion. Solid fluorescence was observed for these crystals with a broad emission from 600 nm to longer than 850 nm. The CVA results correspond to those for known substituted BODIPYs except for the unusually high current observed for the oxidation process of the dithienyl derivatives with respect to the reduction process. This finding indicates oxidative film deposition.


 Please wait while we load your content...
Please wait while we load your content...