An ion-pair receptor comprising urea groups and N-benzyl-aza-18-crown-6: effective recognition and liquid–liquid extraction of KCl salt†
Abstract
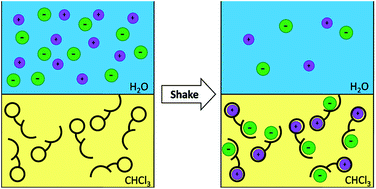
Compound 1 was designed and prepared as a heteroditopic ion-pair receptor that contains two urea groups for anion complexation and N-benzyl-18-crown-6 for cation recognition. These ion-binding domains are assembled together by the L-ornithine scaffold. Qualitative cation coordination studies of receptor 1, supported by quantitative data received for monotopic N-(3-nitrobenzyl)-aza-18-crown-6 (3a), have shown that 1 has a strong affinity for Na+, K+ and NH4+ cations. Anion binding studies revealed that in the absence of cations coordinated to 1 (TBA salts), anions are bound with a relatively moderate strength with the selectivity: BzO− > AcO− > Cl−> NO2− > Br−. However, in the presence of cations coordinated to the N-benzyl-18-crown-6 the anion binding affinity increases considerably with the notable exception of carboxylates. For example, chloride binding is increased by over five times in the presence of K+ cations and the selectivity trend for salt binding is: KCl > KOBz > KOAc > KNO2 > KBr. Liquid/liquid extraction studies revealed that receptor 1 is an effective extractant for KCl and NH4Cl salts from aqueous to organic phase.


 Please wait while we load your content...
Please wait while we load your content...