External oxidant-free cross-coupling: electrochemically induced aromatic C–H phosphonation of azoles with dialkyl-H-phosphonates under silver catalysis†
Abstract
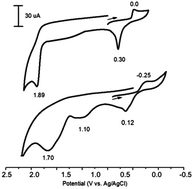
A convenient external oxidant-free method of phosphorylation of azole derivatives (benzo-1,3-azoles, 3-methylindole, 4-methyl-2-acetylthiazole) by using dialkyl-H-phosphonates through the catalytic oxidation of their mixture under electrochemical mild conditions (room temperature, normal pressure) in the presence of silver salts or oxide (1%) is proposed. This method allows us to obtain the desired azole dialkylphosphonates with good yield (up to 75%). The transformations of silver and phosphorus precursors and intermediates using cyclic voltammetry, ESR, and NMR spectroscopy were investigated, and a radical process mechanism was proposed. It has been found that AgP(O)(OEt)2 is oxidized earlier than other components of the reaction mixture with the elimination of a radical. The ESR spectrum of this radical's adduct was obtained in the presence of the radical trap PBN. Ag2+ is out of the catalytic cycle.


 Please wait while we load your content...
Please wait while we load your content...