Abstract
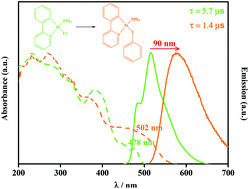
Complexes [Pt(C^N)(PPh3)Cl] (C^N = bzq (7,8-benzoquinolinyl, A) and ppy (2-phenylpyridinyl, B)) were reacted with various thiolate ligands to afford complexes [Pt(C^N)(PPh3)(κ1-S-SR)], C^N = bzq, R = SPh (thiophenolate, 1a); C^N = ppy, R = SPh (1b); C^N = bzq, R = Spy (pyridine-2-thiolate, 2a); C^N = ppy, R = Spy (2b); C^N = bzq, R = SpyN (pyrimidine-2-thiolate, 3a); C^N = ppy, R = SpyN (3b). Complexes 1–3 were characterized by NMR spectroscopy, and the solid-state structures of 1a and 2a were determined by X-ray diffraction methods. Replacing a chloride ligand with electron-rich thiolates changes the lowest energy singlet and triplet excited states to the ones that feature charge transfer from the thiolate (mixed with some metal character) to the C^N ligand, which was supported by TD-DFT calculations. All complexes are emissive at 298 K in the solid state except 2b and 3b, which are emissive only at 77 K having a less rigid structure compared to others. The emission of 1a and 1b originates from a low-energy excited state of dPt/πSR → π*C^N while 3a exhibits a 3LC/3MLCT transition. For 1a and 1b, the radiative rate and the quantum efficiency are higher in a rigid environment such as a solid compared to a polymer and solution. Decreasing the rigidity of the environment leads to a flexibility of rotation of the –SR around the axis of the Pt–S bond. So the geometry can be easily changed after radiation and the lowest lying triplet excited state would have the effective contribution of the dd* transition, which opens a nonradiative pathway at room temperature.


 Please wait while we load your content...
Please wait while we load your content...