Exploring the effect of substituent in the hydrazone ligand of a family of μ-oxidodivanadium(v) hydrazone complexes on structure, DNA binding and anticancer activity†
Abstract
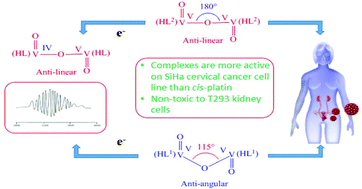
The reaction of 2-hydroxybenzoylhydrazine (H2bh) separately with equimolar amounts of [VIVO(aa)2] and [VIVO(ba)2] in CHCl3 afforded the complexes [VV2O3(HL1)2] (1) and [VV2O3(HL2)2] (2) respectively in good to excellent yield ((HL1)2− and (HL2)2− represent respectively the dianionic form of 2-hydroxybenzoylhydrazones of acetylacetone (H3L1) and benzoylacetone (H3L2) (general abbreviation H3L)). From X-ray structure analysis, the VV–O–VV angle was found to be ∼115° and 180° in 1 and 2 respectively. Upon one-electron reduction selectively at one V centre at an appropriate potential, each of 1 and 2 generated mixed-valence [(HL)VVO-(μ-O)-OVIV(HL)]− species 1A and 2A respectively, which showed valence delocalization at room temperature and localization at 77 K, and the VIV–O–VV bond angles were calculated to be 177.5° and 180° respectively. The intercalative mode of binding of the two complexes 1 and 2 with CT DNA has been suggested by UV-visible spectroscopy (Kb = 7.31 × 105 M−1 and 8.71 × 105 M−1 respectively for 1 and 2), fluorescence spectroscopy (Ksv = 6.85 × 105 M−1 and 8.53 × 105 M−1 respectively for 1 and 2) and circular dichroism spectroscopy. Such intercalative mode of binding of these two complexes with CT DNA and HPV DNA has also been confirmed by molecular docking study. Both complexes 1 and 2 exhibited promising anti-cancer activity against SiHa cervical cancer cells with IC50 values of 28 ± 0.5 μM and 25 ± 0.5 μM respectively for 24 h which is significantly better than that of widely used cisplatin (with IC50 value of 63.5 μM). Nuclear staining experiments reveal that these complexes kill the SiHa cells through apoptotic mode. It is interesting to note that these two complexes are non-toxic to normal T293 cell line. Complex 2 showed higher DNA binding ability with CT DNA and HPV DNA as well as better anti-cancer properties towards SiHa cervical cancer cells in comparison to complex 1, a fact which can be explained by considering the lower energy of LUMO (which favours electron transition from DNA to the metal complex) and also the higher surface area of complex 2 in comparison to complex 1 due to the presence of one extra electron-withdrawing phenyl group in the former.


 Please wait while we load your content...
Please wait while we load your content...