Catalyst free boron carbon bond cleavage and facile formation of five-membered PNBCC heterocycles†
Abstract
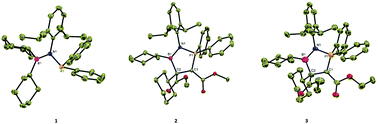
The [3 + 2] cycloaddition reaction of phosphanyl aminoborane [N(2,6-iPr2C6H3)(PPh2)(BCy2)] (1) with activated alkynes led to boron and phosphorus containing five-membered heterocycles [(2,6-iPr2C6H3)NPPh2(CO2R)C–C(Cy)(CO2R)(BCy)] [R = Me (2), Et (3) and H (4)] with facile cleavage of the B–C bond and concomitant formation of a P–C bond with an ylidic character. DFT calculations indicate that 1 can be considered as a non-conjugated 1,3-dipole having two reaction centers viz., a nucleophilic P-center and an electrophilic B-center. The reaction of 1 with the alkynes proceeds through a stepwise dipolar addition mechanism, followed by the migration of the cyclohexyl group from the B-atom to the adjacent C-atom.


 Please wait while we load your content...
Please wait while we load your content...