A robust viologen and Mn-based porous coordination polymer with two types of Lewis acid sites providing high affinity for H2O, CO2 and NH3†
Abstract
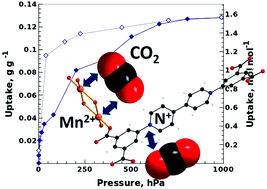
A novel porous coordination polymer [Mn(pc3)(H2O)2]·xH2O (3 < x < 4) is synthesized in water at pH = 7 using the anionic viologen-carboxylate ligand 4,4′-bipyridinium,1,1′-bis-(2,4-dicarboxyphenyl) (pc32−). Dehydration of the material results in the formation of open pores containing two types of accessible Lewis acid sites: exposed Mn2+ cations and N+ atoms of viologen units. Due to this property the PCP shows high affinity and capacity in the adsorption of H2O, CO2 and NH3. Despite the presence of strong adsorption sites this material is stable in liquid water and in gaseous NH3.


 Please wait while we load your content...
Please wait while we load your content...