Synthesis of 13-vertex dimetallacarboranes by electrophilic insertion into 12-vertex ruthenacarboranes†
Abstract
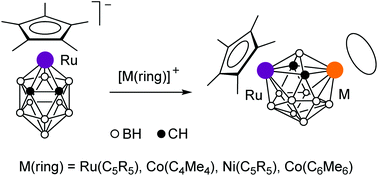
The electrophilic insertion of organometallic species into metallacarboranes was studied in detail for the model compound – the 12-vertex closo-ruthenacarborane anion [Cp*Ru(C2B9H11)]− (1). Reactions of the anion 1 with the 12-electron cationic species [M(ring)]+ (M(ring) = RuCp, RuCp* and Co(C4Me4)) gave the 13-vertex closo-dimetallacarboranes Cp*Ru(C2B9H11)M(ring). Similar reactions of the neutral ruthenacarborane Cp*Ru(Me2S-C2B9H10) produce the cationic dimetallacarboranes [Cp*Ru(Me2S-C2B9H10)M(ring)]+. The symmetrical 13-vertex diruthenacarboranes (C5R5)Ru(R2C2B9H9)Ru(C5R5) can be prepared by the direct reactions of Tl2[7,8-R2-7,8-C2B9H9] (R = H and Me) with two equivalents of [CpRu(MeCN)3]+ or [Cp*RuCl]4. The insertions of the 14-electron cationic species [M(ring)]+ (M(ring) = NiCp, NiCp* and Co(C6Me6)) into 1 gave the 13-vertex dimetallacarboranes Cp*Ru(C2B9H11)M(ring), which have a distorted framework with one open face. The structures of Cp*Ru(C2B9H11)Co(C4Me4) and Cp*Ru(C2B9H11)NiCp were established by X-ray diffraction. Some of the 13-vertex dimetallacarboranes have two electrons less than required by Wade's rules. This violation is explained by the absence of the appropriate pathway for the distortion of the framework.


 Please wait while we load your content...
Please wait while we load your content...