Synthesis, structures and magnetic properties of two chiral mixed-valence iron(ii,iii) coordination networks†
Abstract
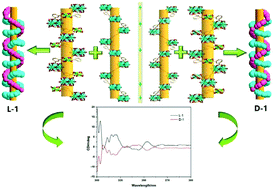
Two rare chiral mixed-valence iron(II,III) coordination networks D-and L-{[FeIIFeIII3O(BTC)3(DEF)3]·0.5H2O}n (D-1 and L-1) (H3BTC = 1,3,5-benzenetricarboxylic acid; DEF = N,N-diethylformamide) have been synthesized without any chiral auxiliary under the solvothermal conditions and structurally characterized by single crystal X-ray crystallography. Structural analysis indicates that these two polymers D-1 and L-1 are enantiomers. The only difference between D-1 and L-1 is that the framework of compound L-1 consists of left-handed double helical chains, while D-1 consists of right-handed double helical chains. Two distinct subunits (SBUs), {(μ3-O)FeIII3(COO)6(DEF)3} and {FeII(COO)6}, are observed in both structures simultaneously. The integration of two distinct SBUs leads to a trinodal (3,3,6)-connected net with an unusual structural topology. Interestingly, despite the achiral nature of H3BTC, the resulting framework exhibits rare chiral helical channels. The experiments show that dodecatungstosilic acid acts as a catalyst which could increase the conversion of the initial reactant. The magnetic studies indicate antiferromagnetic interactions between Fe3+ ions. Additionally, the luminescence studies revealed that the compound exhibited strong photoluminescence emissions at room temperature with a peak at 457 nm, owing to the strong interactions between organic linkers and metal clusters.


 Please wait while we load your content...
Please wait while we load your content...