Amino acid based gallium-68 chelators capable of radiolabeling at neutral pH†
Abstract
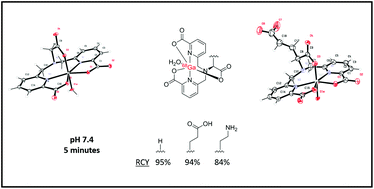
Gallium-68 (68Ga) has been the subject of increasing interest for its potential in the production of radiotracers for diagnosis of diseases. In this work we report the complexation of 68Ga by the amino acid based tripodal chelate H3Dpaa, and two bifunctional derivatives, H3Dpaa.dab and H4Dpaa.ga, under a range of conditions with particular emphasis on the rapid complexation of 68Ga at pH 7.4. 100 μM H3Dpaa achieved a radiochemical yield of 95% at pH 7.4 in 5 minutes at 37 °C. The bifunctional derivatives H4Dpaa.ga and H3Dpaa.dab achieved 94% and 84% radiochemical yields, respectively, under the same conditions. The resulting Ga(III) complexes show thermodynamic stabilities of log KGaDpaa = 18.53, log KGaDpaa.dab = 22.08, log KGaDpaa.ga = 18.36. Unfortunately, the resulting radiolabelled species do not present sufficient serum stability for in vivo application. Herein we show a flexible synthesis for bifunctional chelators based on amino acids that rapidly complex 68Ga under physiological conditions.


 Please wait while we load your content...
Please wait while we load your content...