Synthesis and ORR electrocatalytic activity of mixed Mn–Co oxides derived from divalent metal-based MIL-53 analogues†
Abstract
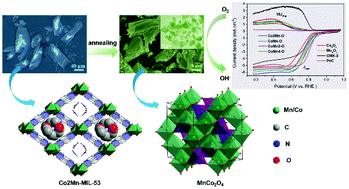
The design of efficient mixed Mn–Co oxides as oxygen reduction reaction (ORR) electrocatalysts in alkaline media has been boosted nowadays. Recently, multivariant MOFs have been demonstrated as versatile precursors to construct these mixed metal oxides. Herein, we synthesized four mixed Mn–Co oxide samples via simply annealing their MIL-53 precursors with different Co/Mn molar ratios. These four samples were dominated by three kinds of Mn–Co spinel phases. The mixed Mn–Co oxides showed enhanced ORR performances compared with the single metal counterparts in alkaline media. In particular, the sample containing a pure MnCo2O4 phase exhibited the highest activity with a half-wave potential of 0.772 V (vs. RHE), only 40 mV negative compared with that of commercial Pt/C. The H2O2 yield was calculated to be below 12.1%, corresponding to the high diffusion-limited current density along with the high electron transfer number. The surface defects, active Mn4+/Mn3+ and electrochemically active surface area together affected the ORR performances of these mixed Mn–Co oxides. The present work highlights the facile controllability of divalent MIL-53 analogues for highly efficient mixed Mn–Co oxides in ORR electrocatalysis.


 Please wait while we load your content...
Please wait while we load your content...