In situ neutron diffraction study of the formation of Ho2Ge2O7 pyrochlore at high temperature and pressure†
Abstract
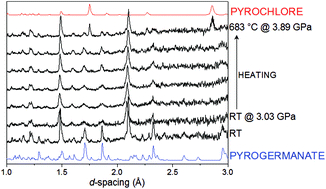
The formation of the spin-ice pyrochlore Ho2Ge2O7 by two different high temperature, high pressure routes has been explored using in situ neutron diffraction. The first route involves the solid-state reaction of Ho2O3 and GeO2, and formation of the pyrochlore phase is observed at 994(27) °C and 3.81(2) GPa, which are significantly milder conditions than those previously reported. The second route involves the hydrothermal synthesis of the tetragonal Ho2Ge2O7 pyrogermanate from Ho(NO3)3·5H2O and GeO2 and its subsequent transformation to the pyrochlore phase, which is observed at 683(23) °C and 3.89(3) GPa. The lowering of the formation temperature of high pressure phases by employment of a precursor of appropriate stoichiometry may also have applications in the wider field of solid-state chemistry.


 Please wait while we load your content...
Please wait while we load your content...