Half-sandwich complexes of Ir(iii), Rh(iii) and Ru(ii) with the MaxPhos ligand: metal centred chirality and cyclometallation†
Abstract
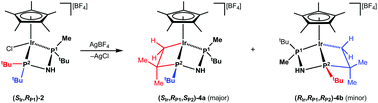
The reaction of the acetylacetonates [(η5-C5Me5)M(acac)Cl] with (SP)-[HMaxPhos][BF4] afforded cationic complexes with the formula (SM,RP)-[(η5-C5Me5)MCl(MaxPhos)][BF4] (M = Rh (1), Ir (2)). The reaction of (SP)-MaxPhos with [RuCl(μ-Cl)(η6-p-MeC6H4iPr)]2 and NH4X afforded (SRu,RP)-[(η6-p-MeC6H4iPr)RuCl(MaxPhos)][X] (X = BF4 (3), PF6 (3′)). The complexes have been completely characterized by analytical and spectroscopic means, including the determination of the crystal structures of 1, 2 and 3′. Treatment of the iridium complex 2 with AgBF4, at 253 K, resulted in the intramolecular cyclometallation of one of the tert-butyl substituents of the MaxPhos diphosphane ligand, affording a mixture of isomers of (SIr,RP1,SP2 and RIr,RP1,RP2)-[(η5-C5Me5)Ir(MaxPhos)][BF4] (4a and 4b). However, rhodium complex 1 and ruthenium complex 3 reacted with AgBF4 forming the expected unsaturated intermediates “(ηn-ring)M(MaxPhos)” which were trapped by MeCN, affording the cationic adducts (SM,RP)-[(ηn-ring)M(MaxPhos)(MeCN)][BF4]2 (M = Rh (5), Ru (6)). Only one epimer at the metal was isolated in high yield for the complexes 1, 2, 3, 3′, 5 and 6 and the metallation of 2 to give 4 occurs with high diastereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...