Mg doped Li2FeSiO4/C nanocomposites synthesized by the solvothermal method for lithium ion batteries
Abstract
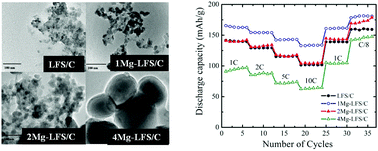
A series of porous Li2Fe1−xMgxSiO4/C (x = 0, 0.01, 0.02, 0.04) nanocomposites (LFS/C, 1Mg-LFS/C, 2Mg-LFS and 4Mg-LFS/C) have been synthesized via a solvo-thermal method using the Pluronic P123 polymer as an in situ carbon source. Rietveld refinement of the X-ray diffraction data of Li2Fe1−xMgxSiO4/C composites confirms the formation of the monoclinic P21 structure of Li2FeSiO4. The addition of Mg facilitates the growth of impurity-free Li2FeSiO4 with increased crystallinity and particle size. Despite having the same percentage of carbon content (∼15 wt%) in all the samples, the 1Mg-LFS/C nanocomposite delivered the highest initial discharge capacity of 278 mA h g−1 (∼84% of the theoretical capacity) at the C/30 rate and also exhibited the best rate capability and cycle stability (94% retention after 100 charge–discharge cycles at 1C). This is attributed to its large surface area with a narrow pore size distribution and a lower charge transfer resistance with enhanced Li-ion diffusion coefficient compared to other nanocomposites.


 Please wait while we load your content...
Please wait while we load your content...