μ-Pyridine-bridged copper complex with robust proton-reducing ability†
Abstract
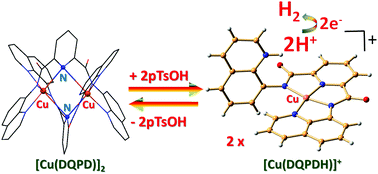
The binuclear copper complex [Cu(DQPD)]2 (where DQPD = deprotonated N2,N6-di(quinolin-8-yl)pyridine-2,6-dicarboxamide (DQPDH2)) was synthesised and characterised by various spectroscopic as well as electrochemical techniques. The binuclear copper complex was converted into a mononuclear one by the addition of 2 equivalents of pTsOH into [Cu(DQPD)]2. The interconversion between the dimer and monomer complex was studied through UV-Vis spectroscopy and cyclic voltammetry. The mononuclear copper complex showed high catalytic activity towards electrochemical proton reduction using acetic acid as the external proton source in 95 : 5 (v/v) DMF/H2O. It showed an ic/ip (where ic is the catalytic current in the presence of acetic acid and ip is the reduction peak current in absence of acid) value of 24 and a turnover rate (TOF) of 111.70 s−1 at a scan rate of 100 mV s−1 at 25 °C. The [Cu(DQPD)]2 complex evolved hydrogen under the irradiation of visible light in the presence of fluorescein (Fl) as a photosensitizer and triethylamine (TEA) as the sacrificial electron donor with an initial TOF of 0.03 s−1 with respect to the catalyst.


 Please wait while we load your content...
Please wait while we load your content...