Pushing the photodelivery of nitric oxide to the visible: are {FeNO}7 complexes good candidates?†
Abstract
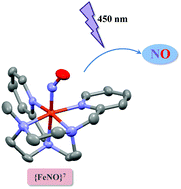
Photodelivery of NO requires stable compounds which can be made reactive by irradiation with (visible) light. Traditional {MNO}6 complexes require a substantial ligand design to shift their absorption spectra to the appropriate region of the electromagnetic spectrum. [Fe((CH2Py2)2Me[9]aneN3)(NO)](BF4)2 is a new {FeNO}7 octahedral coordination compound, which is thermally and air-stable in solution. Illumination with a 450 nm light source induces significant photodetachment of the coordinated NO (ϕNO = 0.52 mol einstein−1), suggesting that {FeNO}7 compounds can be in fact suitable compounds for therapeutic NO-photorelease.


 Please wait while we load your content...
Please wait while we load your content...