Comparison of the Ca2+ complexing properties of isosaccharinate and gluconate – is gluconate a reliable structural and functional model of isosaccharinate?†
Abstract
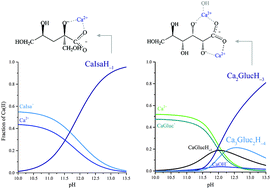
The calcium complexation and acid–base properties of α-D-isosaccharinate (Isa−) in neutral and in (hyper)alkaline solutions have been investigated via potentiometric titrations, multinuclear NMR, ESI-MS and quantum chemical calculations. Isa− is the primary alkaline degradation product of cellulose, and may be present in radioactive waste repositories and therefore, it could contribute to the mobilization of radioactive nuclei. Because of its limited availability, D-gluconate (Gluc−) is commonly used as a structural and functional model of Isa−. Therefore, the thermodynamic and structural data obtained for Isa− were compared with those of Gluc−. The formation constants of the CaIsa+ and CaGluc+ complexes present in neutral solutions are practically identical, but the binding sites are in different positions and the CaIsa20 solution species cannot be detected. The stepwise formation constant of the CaIsaH−10 complex (forming in alkaline medium) is somewhat larger than that of CaGlucH−10, which is in line with the observation that IsaH−12− is a stronger base than GlucH−12−. The most striking difference is that, unlike Gluc−, Isa− does not form polynuclear complexes with Ca2+. The structural reason for this is that the alcoholate groups on C2 and C3 adjacent to the carboxylate moiety on Gluc− are able to simultaneously bind Ca2+, making the formation of polynuclear Ca-complexes possible. On Isa−, only the alcoholate on C2 is involved, while the other one on C6 is not (supposedly for steric reasons). In conclusion, during the interactions of Gluc− and Isa− with Ca2+, differences rather than similarities prevail.


 Please wait while we load your content...
Please wait while we load your content...