Dimer formation of GdDO3A-arylsulfonamide complexes causes loss of pH-dependency of relaxivity†
Abstract
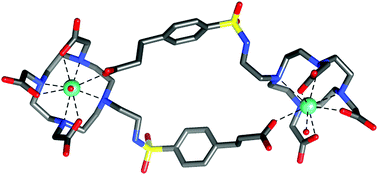
Gadolinium(III) complexes with pH-dependent relaxivities have been proposed as responsive magnetic resonance imaging (MRI) contrast agents (CA) for mapping of pH value in living subjects. The latter is clinically relevant because hypoxia-induced reduction of interstitial pH (acidosis) is a hallmark of tumor progression and resistance against chemotherapy. In order to obtain versatile building blocks for integration of a pH-responsive MRI-CA functionality into larger molecular assemblies, such as bioconjugates, micelles or nanoparticles, we equipped the structural motif GdDO3A-ethylene(arylsulfonic acid) with additional carboxylic acid moieties in the aromatic para-position. Two novel compounds were characterized concerning their pH-dependent relaxivity as well as by 17O NMR and 1H NMRD, augmented by determination of luminescence lifetimes of the respective Eu(III) complexes and structural modelling by density functional theory (TPSSh/LCRECP/6-31G(d)). Unexpected involvement of the peripheral carboxylates into metal ion complexation effected self-assembly of the lanthanide(III) complexes, resulting in dimeric species comprising two lanthanide ions, two symmetrically bridging ligands, and one coordinated water molecule per Gd(III) (q = 1). These structures are stable even at low concentrations and in presence of competing anions like phosphate. The pH-sensitive sulfonamide moieties are not involved into Gd(III) coordination, resulting in virtually constant relaxivities of r1 = 6.7 mM−1 s−1 (298 K, 20 MHz) over the biologically relevant pH range (4 to 9). Since further functionalisation on the peripheral carboxylates would effectively inhibit dimer formation, the compounds are nonetheless suited for the initially envisaged field of application.


 Please wait while we load your content...
Please wait while we load your content...