Photophysical properties of three coordinated copper(i) complexes bearing 1,10-phenanthroline and a monodentate phosphine ligand†
Abstract
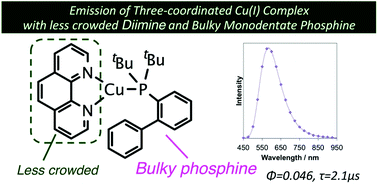
We report the synthesis and photophysical properties of Cu(I) complexes with 1,10-phenanthroline (phen) and monodentate phosphine ligands. Single crystal X-ray structural analysis revealed that these have three-coordinated trigonal planar geometries. We also found that one of them, [Cu(phen)(Johnphos)]BF4 (Johnphos = 2-(di-tert-butylphosphino)biphenyl), is considerably emissive both in solution and solid states. The emission maximum wavelength of the emission of the complex is 580 nm, and the lifetime of the emission is 2 μs in solution. Moreover, we have systematically investigated the photophysical and redox properties of four-coordinate complexes [Cu(NN)(P)2]+ in addition to three-coordinate complexes [Cu(NN)(P)]+. Charge transfer transitions play a key role in the photophysics of these complexes.


 Please wait while we load your content...
Please wait while we load your content...