Theoretical investigation of the impact of ligands on the regiodivergent Rh-catalyzed hydrothiolation of allyl amines†
Abstract
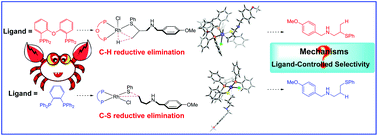
The reaction mechanisms of the Rh-catalyzed regiodivergent hydrothiolation of allyl amines have been theoretically investigated with the aid of density functional theory (DFT) calculations. The impact of ligands on the regioselectivity was rationalized. The origin of the regioselectivity involved in these reactions was probed by investigating the electronic and steric effects. The regioselectivity is derived from the stability of related intermediates. Among them, the steric effect plays an important role in controlling the regioselectivity. The origin of the regioselectivity could also be interpreted through the electronic effect on the corresponding transition states.


 Please wait while we load your content...
Please wait while we load your content...