(C^Npz^C)AuIII complexes of acyclic carbene ligands: synthesis and anticancer properties†
Abstract
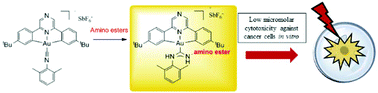
A series of cyclometallated gold(III) complexes supported by pyrazine-based (C^Npz^C)-type pincer ligands were synthesized via two different pathways. Nucleophilic attack on the isocyanide complex [(C^Npz^C)Au(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NC6H3Me2-2,6)]SbF6 (2) gave [(C^Npz^C)Au(ACC)]SbF6 complexes with aniline (4·SbF6), adamantylamine (5), glycine ethyl ester (6), alanine methyl ester (7), valine methyl ester (8), phenylglycine methyl ester (9) and methionine methyl ester (10) substituents (ACC = acyclic carbene). The pathway via isocyanide insertion into gold–amide bonds was also investigated; e.g. the reaction of xylyl isocyanide with (C^Npz^C)AuNHPh followed by protonation with HBF4·OEt2 gave the acyclic carbene complex 4·BF4. To the best of our knowledge compounds 6–10 represent the first examples of gold(III) acyclic carbene complexes bearing amino acid functions. The compounds provide a versatile platform for the study of the anti-proliferative properties of gold(III) complexes. Tests against human adenoma-type lung cancer cells identified 5, 6, 7 and 10 as particularly promising and demonstrate the synthetic flexibility of acyclic carbene complexes and the potential of that class of compounds for anticancer applications. Compared to cisplatin, amino ester-containing ACC complexes showed improved selectivity for MCF-7 breast cancer cells over that for healthy fibroblasts.
NC6H3Me2-2,6)]SbF6 (2) gave [(C^Npz^C)Au(ACC)]SbF6 complexes with aniline (4·SbF6), adamantylamine (5), glycine ethyl ester (6), alanine methyl ester (7), valine methyl ester (8), phenylglycine methyl ester (9) and methionine methyl ester (10) substituents (ACC = acyclic carbene). The pathway via isocyanide insertion into gold–amide bonds was also investigated; e.g. the reaction of xylyl isocyanide with (C^Npz^C)AuNHPh followed by protonation with HBF4·OEt2 gave the acyclic carbene complex 4·BF4. To the best of our knowledge compounds 6–10 represent the first examples of gold(III) acyclic carbene complexes bearing amino acid functions. The compounds provide a versatile platform for the study of the anti-proliferative properties of gold(III) complexes. Tests against human adenoma-type lung cancer cells identified 5, 6, 7 and 10 as particularly promising and demonstrate the synthetic flexibility of acyclic carbene complexes and the potential of that class of compounds for anticancer applications. Compared to cisplatin, amino ester-containing ACC complexes showed improved selectivity for MCF-7 breast cancer cells over that for healthy fibroblasts.


 Please wait while we load your content...
Please wait while we load your content...