Selective formation of discrete versus polymeric copper organophosphates: DNA cleavage and cytotoxic activity†
Abstract
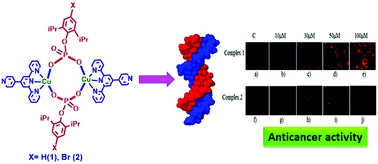
Copper phosphate metalloligands [Cu(X-dipp)(Pyterpy)]2 [X = H (1), Br (2)], exemplifying expanded 4,4′-bipyridine type molecules, have been synthesized by reacting 4′-(4-pyridyl)-2,2′:6′,2′′-terpyridine (Pyterpy) and para substituted 2,6-diisopropylphenyl phosphate (X-dippH2) with copper acetate. The pendant N,N-ends of dimeric copper phosphates 1 and 2 have been forced to engage in further coordination by limiting the concentration of Pyterpy in the reaction mixture to yield rare Pyterpy bridged corner-shared polymeric copper phosphates [Cu2(X-dippH)(X-dipp)(Pyterpy)(H2O)]n [X = Cl (3), Br (4), I (5)]. The formation of 1–5 is supported by spectroscopic and analytical data. The solid state structures of these compounds have further been confirmed by single-crystal X-ray diffraction studies. Soluble dimeric complexes 1 and 2 have been assessed for their in vitro anti-tumour properties against human breast and colorectal cancer cell lines. The DNA cleavage, protein cleaving and cytotoxicity assays revealed that these compounds are effective in cleaving DNA, while the activity of 1 as an anti-tumor agent is better than 2.


 Please wait while we load your content...
Please wait while we load your content...