Sonogashira (Cu and amine free) and Suzuki coupling in air catalyzed via nanoparticles formed in situ from Pd(ii) complexes of chalcogenated Schiff bases of 1-naphthaldehyde and their reduced forms†
Abstract
The reaction of 1-naphthaldehyde with 2-(phenylthio/seleno)ethylamine afforded air- and moisture-insensitive Schiff bases: C10H7-1-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-CH2CH2EPh (L1: E = S; L2: E = Se). Then, on treatment with NaOAc and Li2PdCl4, palladacycles, [Pd(L1-H/L2-H)Cl] (1/2) were formed at room temperature, in which L1/L2 are ligated as an unsymmetric (C−, N, E) pincer. The reduction of >C
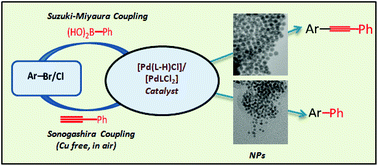
N-CH2CH2EPh (L1: E = S; L2: E = Se). Then, on treatment with NaOAc and Li2PdCl4, palladacycles, [Pd(L1-H/L2-H)Cl] (1/2) were formed at room temperature, in which L1/L2 are ligated as an unsymmetric (C−, N, E) pincer. The reduction of >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds of L1 and L2 with sodium borohydride gave C10H7-1-CH2NH-CH2CH2EPh (L3: E = S; L4: E = Se). The reactions of L3/L4 at room temperature, similar to those of L1/L2, resulted in the formation of complex [Pd(L3/L4)Cl2] (3/4), in which the ligand is coordinated in a bidentate (N, E) mode. The yield of all the complexes was >85%. Characterization by HR-MS, 1H, 13C{1H} and 77Se{1H} NMR spectra of L1–L4 and their complexes 1–4 were performed. The structures of L1 and 1–4 were established with single-crystal X-ray diffraction. In all the complexes, the geometry of palladium was distorted square planar. The Pd–S bond distances in 1 and 3 were 2.426(12) and 2.259(2) Å, respectively, whereas Pd–Se bond lengths (Å) were 2.523(11) (2) and 2.369(10) (4) Å. The catalytic activities of 1–4 were explored for copper- and amine-free Sonogashira and Suzuki–Miyaura coupling (SMC) of aryl halides under aerobic conditions. The amount of catalyst required for achieving good conversion was 0.01 and 0.05 mol% for SMC and Sonogashira coupling, respectively. The conversion of some substrates reached a maximum in 1 and 2 h for Sonogashira coupling and SMC, respectively. The palladacycles as catalysts gave good conversion efficiency. The generation of palladium-containing nanoparticles (NPs) during both coupling reactions was observed. These were isolated and HR-TEM studies were performed on them and revealed their size as ∼2–7 nm. The SEM-EDX analysis indicated the presence of organochalcogen ligands or their fragments in the samples. They independently catalyzed both reactions. Therefore, the role of 1–4 in catalysis undoubtedly exists. For Sonogashira coupling, the formation and role of such Pd-based NPs under aerobic conditions were observed for the first time. The complexes 1–4 showed the potential for reuse, as in the eighth cycle, conversion dropped by only 20%.
N bonds of L1 and L2 with sodium borohydride gave C10H7-1-CH2NH-CH2CH2EPh (L3: E = S; L4: E = Se). The reactions of L3/L4 at room temperature, similar to those of L1/L2, resulted in the formation of complex [Pd(L3/L4)Cl2] (3/4), in which the ligand is coordinated in a bidentate (N, E) mode. The yield of all the complexes was >85%. Characterization by HR-MS, 1H, 13C{1H} and 77Se{1H} NMR spectra of L1–L4 and their complexes 1–4 were performed. The structures of L1 and 1–4 were established with single-crystal X-ray diffraction. In all the complexes, the geometry of palladium was distorted square planar. The Pd–S bond distances in 1 and 3 were 2.426(12) and 2.259(2) Å, respectively, whereas Pd–Se bond lengths (Å) were 2.523(11) (2) and 2.369(10) (4) Å. The catalytic activities of 1–4 were explored for copper- and amine-free Sonogashira and Suzuki–Miyaura coupling (SMC) of aryl halides under aerobic conditions. The amount of catalyst required for achieving good conversion was 0.01 and 0.05 mol% for SMC and Sonogashira coupling, respectively. The conversion of some substrates reached a maximum in 1 and 2 h for Sonogashira coupling and SMC, respectively. The palladacycles as catalysts gave good conversion efficiency. The generation of palladium-containing nanoparticles (NPs) during both coupling reactions was observed. These were isolated and HR-TEM studies were performed on them and revealed their size as ∼2–7 nm. The SEM-EDX analysis indicated the presence of organochalcogen ligands or their fragments in the samples. They independently catalyzed both reactions. Therefore, the role of 1–4 in catalysis undoubtedly exists. For Sonogashira coupling, the formation and role of such Pd-based NPs under aerobic conditions were observed for the first time. The complexes 1–4 showed the potential for reuse, as in the eighth cycle, conversion dropped by only 20%.


 Please wait while we load your content...
Please wait while we load your content...