A new glance on R2MGe6 (R = rare earth metal, M = another metal) compounds. An experimental and theoretical study of R2PdGe6 germanides†
Abstract
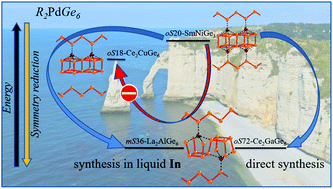
The R2PdGe6 series (R = rare earth metal) was structurally characterized, and the results achieved were extended for a comprehensive study on R2MGe6 (M = another metal) compounds, employing symmetry-based structural rationalization and energy calculations. Directly synthesized R2PdGe6 exists for almost all R-components (R = Y, La–Nd, Sm and Gd–Lu) and even if with La is probably metastable. Several single crystal X-ray analyses (R = Y, Ce, Pr, Nd, Er and Lu) indicated oS72-Ce2(Ga0.1Ge0.9)7 as the correct structure. The alternative In-flux method, once optimized, produced three good quality R2PdGe6 single crystals: La2PdGe6 and Pr2PdGe6 turned out to be mS36-La2AlGe6-type non-merohedrally twinned crystals and Yb2PdGe6 is of oS72-Ce2(Ga0.1Ge0.9)7-type. The vacancy ordering phenomenon was considered as a possible cause of the symmetry reduction relations connecting the most frequently reported 2 : 1 : 6 structural models (oS18, oS72 and mS36) with the oS20-SmNiGe3 aristotype. The detected twin formation is consistent with the symmetry relations, which are discussed even considering the validity of the different structural models. DFT total energy calculations were performed for R2PdGe6 (R = Y and La) in the three abovementioned structural models, and for La2MGe6 (M = Pt, Cu, Ag and Au) in the oS18 and oS72 modifications. The results indicate that the oS18-Ce2CuGe6 structure, prevalently proposed in the literature, is associated with the highest energy and thus it is not likely to be realized in these series. The oS72 and mS36 polytypes are energetically equivalent, and small changes in the synthetic conditions could easily stabilize any of them, in agreement with experimental results obtained by direct and flux syntheses.


 Please wait while we load your content...
Please wait while we load your content...