Iron(ii) complexes of 2-mercaptopyridine as rubredoxin site analogues†
Abstract
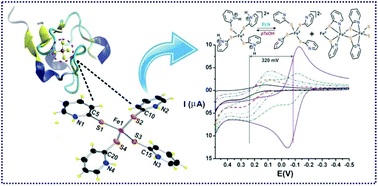
The synthesis, characterization and reactivity studies of iron(II) complexes [FeII(PySH)4](OTf)2, 1-(OTf)2, [FeII(PySH)4](ClO)4, 1-(ClO4)2, and [FeII(PyS)2]n, (2), of a 2-mercaptopyridine (PySH) ligand are discussed. The X-ray crystal structures of both 1-(OTf)2 and 1-(ClO4)2 reveal a distorted tetrahedral geometry at the iron(II) center with identical constituents. All the pyridine nitrogen atoms are protonated and thiolate ions are coordinated to the iron(II) center. The structure and function of complex 1-(OTf)2 or 1-(ClO4)2 resembles the active site of rubredoxin. Complex 2 has octahedral geometry at the iron(II) center forming a 1-D coordination polymer. Complex 1-(OTf)2 exhibits a high positive redox potential (E1/2 = 0.23 V vs. Ag/AgCl) which reduces to −0.12 V in the presence of triethylamine under an inert atmosphere. This change of the redox potential is highly reversible in the presence of a weak acid such as p-toluenesulfonic acid, pTsOH. DFT studies show that the complex cation [FeII(PySH)4]2+ upon treatment with a base converts to its anionic congener, [FeII(PyS)4]2−, via the deprotonation of the pyridinium moiety. The iron(II) complexes readily react with molecular oxygen to yield the corresponding iron(III) complex, which rapidly decays to form pyridine disulphide (Py2S2) and an iron(II) complex.


 Please wait while we load your content...
Please wait while we load your content...