Experimental and ab initio studies of Cd5(BO3)3Cl: the first cadmium borate chlorine NLO material with isolated BO3 groups†
Abstract
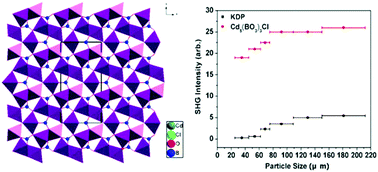
A nonlinear optical crystal cadmium borate chlorine (Cd5(BO3)3Cl) has been successfully grown through a spontaneous crystallization method. Cd5(BO3)3Cl crystallizes in the noncentrosymmetric space group Cm with isolated BO3 and distorted CdOnClm (n = 4, 5, 7; m = 0, 1, 2) polyhedra. Powder second harmonic generation (SHG) measurements on polycrystalline Cd5(BO3)3Cl indicated that the title compound is phase-matchable in the visible region and exhibits a large SHG response of about 5 × KH2PO4 (KDP). Further optical characterization suggested that the compound has a wide transparent region ranging from UV to near IR with a UV cutoff edge at about 295 nm. In addition, first-principles electronic structure calculations revealed that the macroscopic SHG coefficients of Cd5(BO3)3Cl originate from the cooperative effects of the BO3 groups with asymmetric π-delocalization, the d10 cation Cd2+ with the polar displacement and the Cl− anions.


 Please wait while we load your content...
Please wait while we load your content...