Room temperature transmetallation from tris(pentafluorophenyl)borane to cyclometallated iridium(iii)†
Abstract
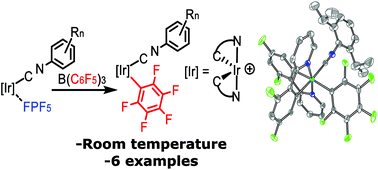
Transmetallation reactions involving organoboron reagents and transition metals are legion in synthetic organometallic chemistry and homogeneous catalysis. Triarylboranes (BAr3) have been observed to participate in transmetallation reactions with many transition metals, typically following abstraction of an alkyl (R−) or hydride ligand by the Lewis acidic borane. Here, an unusual transmetallation strategy is described where an aryl group from a borane replaces a weakly coordinated PF6− ligand. The precursors Ir(C^N)2(CNAr)(FPF5) (C^N = cyclometallating ligand, CNAr = aryl isocyanide) react smoothly with B(C6F5)3 to give complexes of the type Ir(C^N)2(CNAr)(C6F5), a previously unobserved structure type featuring an unchelated aryl ligand. The reaction tolerates a variety of C^N ligands and a range of electronically and sterically varied aryl isocyanide ancillary ligands. A total of six complexes of this type are described, two of which are characterized by single-crystal X-ray diffraction. All but one of the complexes luminesces at room temperature, with the emission wavelength dependent on the C^N ligand.


 Please wait while we load your content...
Please wait while we load your content...