Why different ligands can control stereochemistry selectivity of Ni-catalyzed Suzuki–Miyaura cross-coupling of benzylic carbamates with arylboronic esters: a mechanistic study†
Abstract
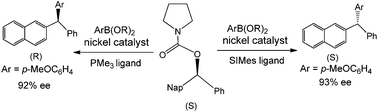
Quantum chemistry calculations have been employed to study the mechanisms of nickel-catalyzed Suzuki–Miyaura cross-coupling reactions of benzylic carbamates with arylboronic esters, and the energy profiles have been computed to evaluate possible origins for the generation of different stereochemistry products. It has been demonstrated that the mechanism can be divided into three steps: oxidative addition, transmetallation and reduction elimination. Transmetallation is the rate-limiting step for the whole reaction cycle, and oxidative addition controls the stereoselectivity of the resulting products. Two possible pathways for the transmetallation step were proposed to consider the presence and absence of a base, and the results indicated that the former is energetically more favorable. Different ligands of nickel catalysts yield two kinds of stereochemistry products. For a phosphine ligand, an R product is afforded while for the N-heterocyclic carbene ligand, an S product is afforded. The distortion/interaction energy analysis and percent buried volume models have been performed to illustrate the origins of reaction stereoselectivity, and the interactions between catalysts and organic moieties control the stereoselectivity for both Ni(PMe3) and Ni(SIMes) catalysts.


 Please wait while we load your content...
Please wait while we load your content...