Two titanium(iv)-oxo-clusters: synthesis, structures, characterization and recycling catalytic activity in the oxygenation of sulfides†
Abstract
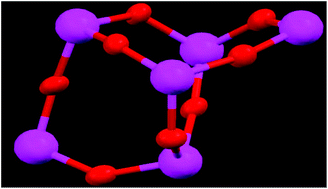
Two 5-sulfosalicylic acid (H3ssal) and 1,10-phenanthroline (Phen) substituted titanium-oxo-clusters, [Ti6O8 (Hssal)2(Phen)6(H2O)4]·4OH·21H2O (TOC-1) and [Ti4O4(Hssal)4(Phen)4]·18H2O·4NH3 (TOC-2), have been synthesized and fully characterized by single crystal X-ray diffraction, FT-IR spectroscopy, elemental analysis, thermogravimetric analysis and fluorescence analysis. The structure of TOC-1 is a hexanuclear cluster containing a {Ti6(μ2-O)8} core, where all titanium ions can be described in a distorted octahedral geometry with both 1,10-phenanthroline and 5-sulfosalicylate serving as chelating ligands, while TOC-2 consists of a tetranuclear {Ti4(μ2-O)4} skeleton forming an approximate plane quadrilateral where both 5-sulfosalicylate and 1,10-phenanthroline ligands chelate to the titanium atom and the oxygen atom bridges two titanium atoms. Moreover, catalytic activity for the selective oxidation of sulfides, methyl phenyl sulfide (MPS) and phenyl sulfide (PPS), has also been explored. The results showed that TOCs 1 and 2 exhibit excellent catalytic performance in the oxidation of sulfides to sulfoxides with H2O2 as an oxidant. In particular, TOC-1 is an efficient homogeneous catalyst and very interestingly it can be recovered by filtration upon cooling and then reused at least four times without losing activity, which is very rare in the oxygenation of organic sulfides.


 Please wait while we load your content...
Please wait while we load your content...