Photoluminescence properties of TADF-emitting three-coordinate silver(i) halide complexes with diphosphine ligands: a comparison study with copper(i) complexes†
Abstract
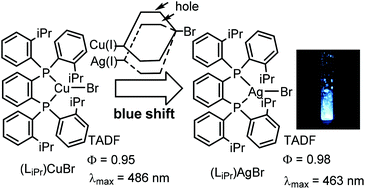
Synthesis and X-ray structures of silver(I) bromide complexes with diphosphine ligands LMe, LEt, and LiPr are described, where LMe = 1,2-bis[bis(2-methylphenyl)phosphino]benzene, LEt = 1,2-bis[bis(2-ethylphenyl)phosphino]benzene, and LiPr = 1,2-bis[bis(2-isopropylphenyl)phosphino]benzene. Crystals of complex [(LMe)AgBr]2 (1), prepared from LMe and AgBr, showed a tetrahedral bimetallic structure. LEt and LiPr, with bulkier substituents than those of LMe, reacted with AgBr to give crystalline three-coordinate complexes (LEt)AgBr (2) and (LiPr)AgBr (3). Nuclear magnetic resonance (NMR) studies demonstrated that 1 dissociates in solution to yield a monomeric three-coordinate complex (LMe)AgBr. Luminescence studies showed that complexes 1–3 exhibit efficient blue thermally activated delayed fluorescence (TADF) in both solid state and solution. Density functional theory (DFT)/Time-dependent (TD)-DFT calculations revealed that the electronic transition responsible for TADF corresponds to (σ + Br) → π*. The photophysical properties of silver complexes 1–3 are discussed in detail and compared to those of the copper complex (LMe)CuBr.


 Please wait while we load your content...
Please wait while we load your content...