Fluorite-type coordination compound as iodide ion conductor: crystal structure and ionic conductivity†
Abstract
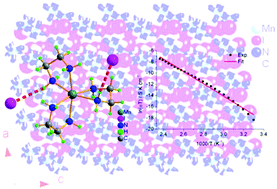
The solid state electrolytes show a wide range of practical applications in a variety of all-solid-state electrochemical devices, and it is highly in demand to explore new types of solid state electrolyte materials. In this study, we have designed and prepared a fluorite-type coordination compound, [Mn(en)3]I2, which has been characterized by microanalysis for C, H and N elements, infrared spectrum in the wavenumber range of 4000–400 cm−1, thermogravimetric analysis and differential scanning calorimetry. The single crystal X-ray diffraction revealed that the bigger size [Mn(en)3]2+ cations build three-dimensional network in the crystal of [Mn(en)3]I2 and the smaller size iodide ions occupy the tetrahedral or octahedral cavities surrounded by the [Mn(en)3]2+ cations, featuring as the fluorite-type compound. The impedance spectra were investigated to reveal the ionic conductivity σ = 3.45 × 10−11 S cm−1 at 303 K, while σ = 1.37 × 10−6 S cm−1 at 423 K, sharply increasing by five orders of magnitude regarding to that at 303 K. The electric modulus analysis further confirmed the conductance contributed from the migration of iodide ions. This study opens a way to design and achieve new coordination compound-based ion conductors.


 Please wait while we load your content...
Please wait while we load your content...