New homoleptic bis(pyrrolylpyridiylimino) Mg(ii) and Zn(ii) complexes as catalysts for the ring opening polymerization of cyclic esters via an “activated monomer” mechanism†
Abstract
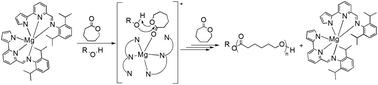
The reaction of MgBu2 and ZnEt2 or Zn{N[Si(CH3)3]2}2 with a tridentate monoanionic pyrrolylpyridiylimino [N−,N,N] proligand gave homoleptic species, as exclusive products, in high yields. The complexes were characterized in solution by 1D and 2D NMR analysis and by single crystal X-ray crystallography. The new homoleptic complexes were tested as initiators in the polymerization of ε-caprolactone and lactide in the presence of an exogenous alcohol. For both complexes, the polymerizations proceed via an “activated monomer” mechanism that, in the case of the magnesium complex, was correlated with the coordinative flexibility of the ligands, resulting in extremely high productivities under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...