Two-dimensional metal NaCu6.3Sb3 and solid-state transformations of sodium copper antimonides†
Abstract
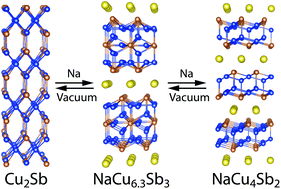
A novel layered compound, NaCu6.3Sb3, has been successfully synthesized from elements. This unique structure crystallizes in the hexagonal space group P63/mmc and has lattice constants of a = 4.2166(2) Å and c = 24.041(1) Å. The structure consists of complex Cu/Sb 2D blocks separated by Na ions. These blocks contain graphene-like hexagonal layers of either Cu or CuSb. The solid-state transformations in the Na–Cu–Sb system between Cu2Sb–NaCu6.3Sb3–NaCu4Sb2 were explored using annealing at different partial vapor pressures of Na, differential scanning calorimetry, and in situ synchrotron powder X-ray diffraction. Full reversibility of the transformation was observed, indicating that the bulk 3D Cu2Sb phase is capable of reversible intercalation of Na ions, forming layered intercalated phases similar to intercalated graphite. The characterization of the transport properties shows that the metallic nature of the electrical conductivity is preserved, even for the Na-rich phase of NaCu4Sb2. Electronic structure calculations support metallic properties in the Cu–Sb layers and predict that no bands cross the Fermi level across the layers, supporting NaCu6.3Sb3 as a two-dimensional metal.


 Please wait while we load your content...
Please wait while we load your content...