C–H addition reactivity of 2-phenylpyridine and 2,2′-bipyridine with pentaphenylborole†
Abstract
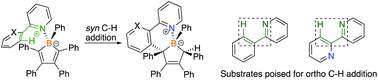
The reactions of pentaphenylborole with pyridines with a pendent aryl group in the 2-position, specifically 2-phenylpyridine and 2,2′-bipyridine, readily afforded adducts. Upon thermolysis, the adducts converted to ortho C–H addition products with the two substituents introduced onto the diene on the boracyclic carbon atoms adjacent to boron in a syn fashion. Photolysis of the syn 2-phenypyridine product led to isomerization to the anti conformer which did not readily occur for the 2,2′-bipyridine complex. Such C–H bond reactivity is uncommon for main group elements and provides insight into methodologies to access boron frameworks.


 Please wait while we load your content...
Please wait while we load your content...