The phosphinoboration of carbodiimides, isocyanates, isothiocyanates and CO2†
Abstract
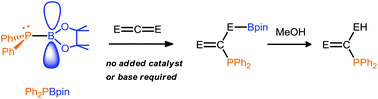
The transition metal-free addition of phosphinoboronate ester Ph2PBpin (pin = 1,2-O2C2Me4) to heterocumulenes including carbodiimides, isocyanates, isothiocyanates and carbon dioxide has been investigated. The corresponding 1,2-addition products were readily prepared at room temperature without the need of a catalyst or added base. Addition of methanol to the compounds derived from addition of Ph2PBpin to carbodiimides, isocyanates, and isothiocyanates resulted in traditional hydrophosphination products. The methodology developed in this study provides a simple and elegant route for the generation of a wide range of functionalized phosphines. The phosphinoboronate ester Ph2PBpin also selectively and reversibly adds to CO2 at room temperature in a 1,2-manner.


 Please wait while we load your content...
Please wait while we load your content...