Two hybrid lanthanide complexes exhibiting a large magnetocaloric effect and slow magnetic relaxation†
Abstract
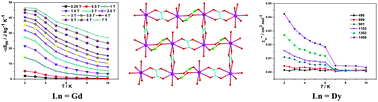
Two isostructural lanthanide (Ln) hybrid complexes co-bridged by organic oxalate and inorganic hypophosphite, [Ln(oxa)(H2PO2)(H2O)2] (oxa = oxalate; Ln = Gd (1), Dy (2)), were solvothermally prepared with the goal of elucidating the role of a hybrid framework in the generation of novel molecular magnetic materials. The title compounds feature a two dimensional (2D) hybrid layer. The LnIII ions are octa-coordinated with distorted square antiprism geometry. The adjacent LnIII ions are co-bridged by hypophosphite and one type of oxalate ligand to form 1D hybrid chains, which are further linked by a second type of oxalate ligand to generate the resulting 2D framework. Magnetic investigations reveal that compound 1 features a large magnetocaloric effect with −ΔSmaxm = 46.60 J kg−1 K−1 (134.39 mJ cm−3 K−1), due to the combined advantages of organic oxalate and inorganic hypophosphite ligands, while compound 2 displays slow magnetic relaxation.


 Please wait while we load your content...
Please wait while we load your content...