A coordination polymer based on dinuclear (pyrazinyl tetrazolate) copper(ii) cations and Wells–Dawson anions for high-performance supercapacitor electrodes†
Abstract
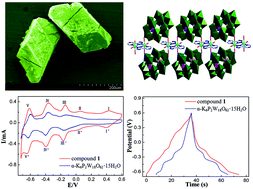
A new coordination polymer, (H2bpe)(Hbpe)2{[Cu(pzta)(H2O)][P2W18O62]}·5H2O (1) (pztaH = 5-(2-pyrazinyl) tetrazolate, bpe = bis(4-pyridyl)ethylene), was synthesized by the hydrothermal method. The structure was determined by single crystal X-ray diffraction analyses and further characterized by the SEM, EDS, BVS, FTIR, and PXRD techniques. In 1, the [P2W18O62]6− (P2W18) clusters as bidentate connectors link [Cu(pzta)(H2O)]24+ dinuclear copper(II) complexes to form inorganic–organic chains. These chains and the [H2bpe]2+ counter-cations are fused together via hydrogen bonding to form a 3D supramolecular architecture. While 1 was employed as the electrode in a supercapacitor, the cycling stability (90.7% capacitance retention after 1000 circles) and specific capacitance (168 F g−1 at a current density of 5 A g−1) of the 1-based electrode are better than those of the parent α-K6P2W18O62-based electrode, demonstrating more outstanding electrochemical performances of 1. Besides, the 1-based electrode showed excellent electrocatalytic activities towards the reduction of H2O2 and KIO3.


 Please wait while we load your content...
Please wait while we load your content...