The role of carbonate in electro-catalytic water oxidation by using Ni(1,4,8,11-tetraazacyclotetradecane)2+†
Abstract
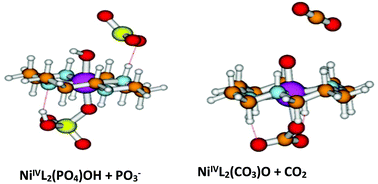
NiLi2+ are good electro-catalysts for water oxidation in phosphate or carbonate buffers. The results point out that the active oxidizing agents are L(X)NiIVOH4−(3−n+1)/(2−n+1), where X = PO4Hn(3−n)− or CO3Hn(2−n)− formed from LNiIVX2via a mechanism involving an acid catalyzed O–P or O–C bond heterolysis. Carbonate behaves differently from phosphate as it is a non-innocent ligand and it can be oxidized.


 Please wait while we load your content...
Please wait while we load your content...